Therapeutic Expertise
With two decades of experience while working with clinical development Biomapas has gathered experience in numerous therapeutic areas throughout various stages of pharmaceuticals, biologicals and medical devices development lifecycle. Our clinical expertise ranges from first-in-human studies all the way up to the delivery of the final study results. Biomapas clinical experts have faced the challenge while helping to bring better cures to the market for numerous different indications in oncology, hematology, cardiology, infectious diseases, gastroenterology, endocrinology, rheumatology, allergology and immunology.
While working in the field coherently for almost twenty years Biomapas Clinical Operations team has established a solid network of close relationship and collaborations with numerous sites and investigators across the whole continent, therefore we are able to support sponsors while selecting and then connecting to the most suitable locations for your trial with the smoothest recruitment potential for almost any therapeutic indication.

Oncology

Rheumatology

Respiratory

Neurology &
Psychiatry

Infectious
Disease

Gynecology
Our areas of expertise
Biomapas expertise spans most therapeutics areas and we have completed Clinical Trials for a broad variety of treatments. However, the competitive field for oncology research is high, with personalized treatments and increasingly complex trial design. Especially in this area, Biomapas has the internal resources to take your product through clinical development and toward commercial success.
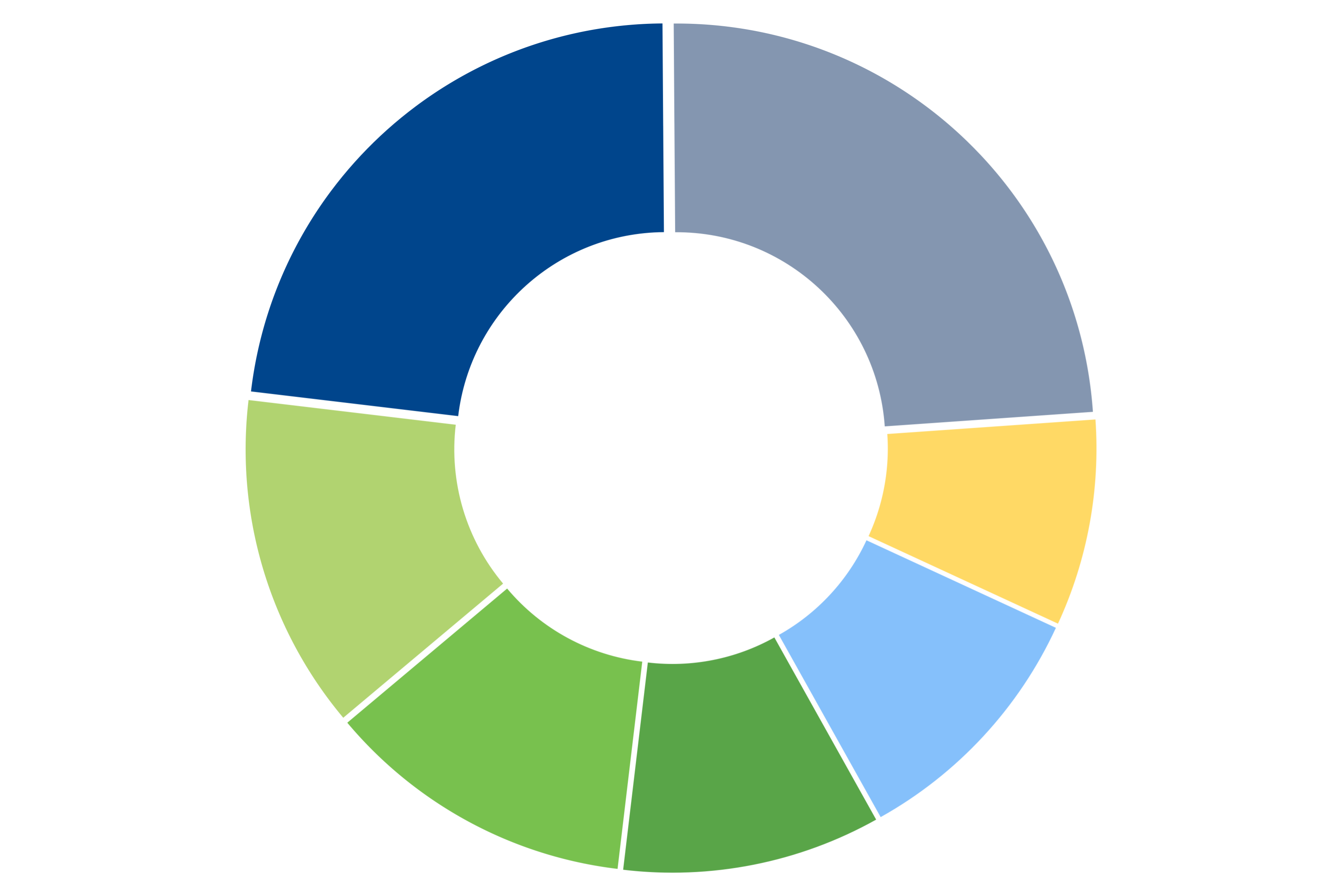
Oncology 23 %
Psychiatry / Neurology 13%
Cardiology 12%
Other 26%
Infectious Disease 10%
Gastroenterology 10%
Endocrinology 8%
Feel free to request information on specific trials and find out how Biomapas can help.
Contact us to learn about how we can accelerate your clinical development, accelerate your path to Market Authorization, and ensure compliance with local and global Post-Marketing requirements.











