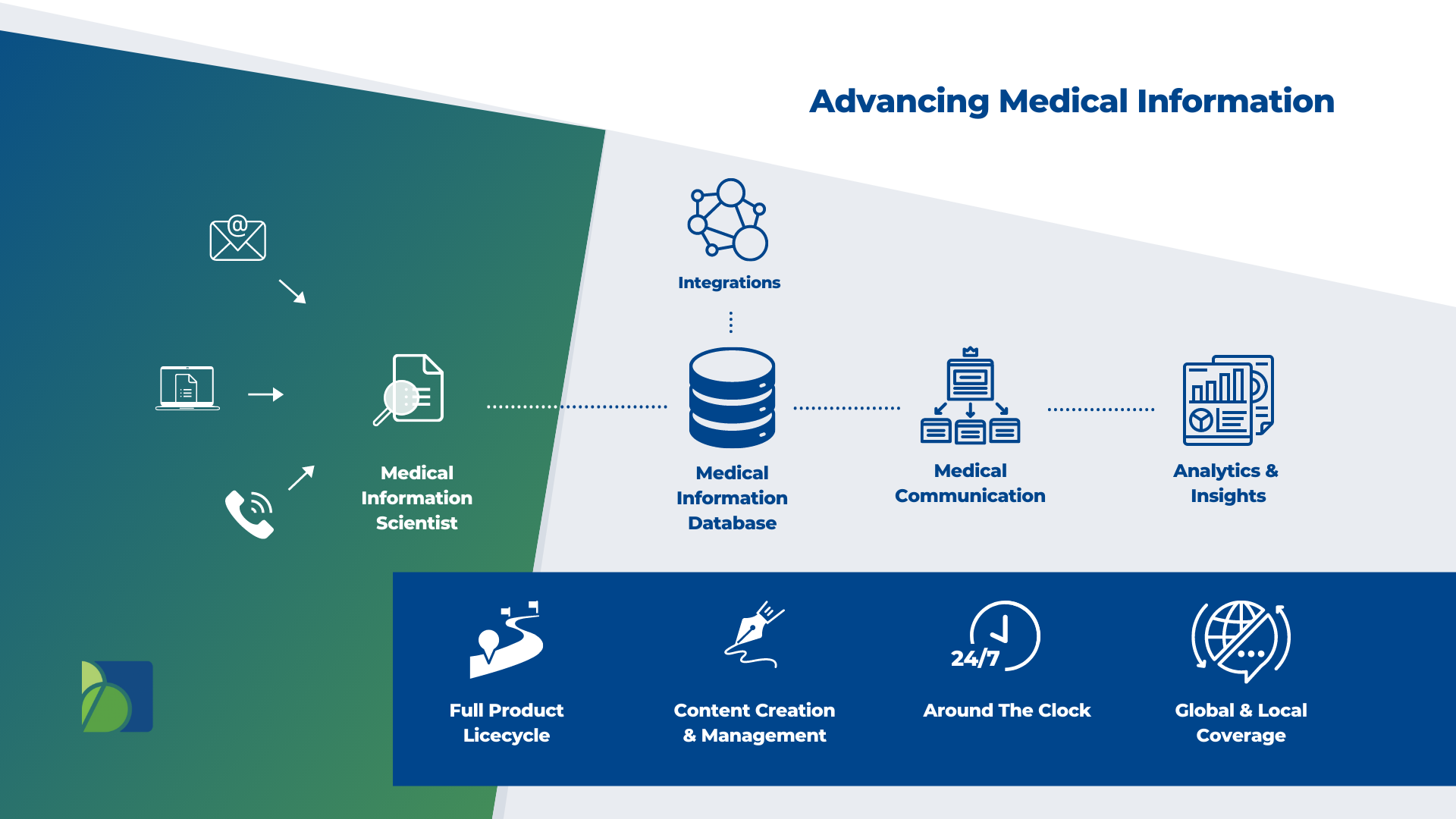
Medical Information is more than responding to unsolicited requests; It is the direct contact point with your brand and an essential part of your communication strategy. At Biomapas, we help you to provide accurate, scientifically-balanced and consistent medical information globally. Whether you need standalone services or integrated support with pharmacovigilance, our experienced team and advanced technology ensure that your medical information is handled professionally and efficiently.
Medical Information Scientist
A 24-hour Medical Information Contact Center is accessible to Biomapas customers seven days a week, 365 days per year. Additionally, our workforce comprises seasoned healthcare professionals and life science graduates who act as an extension of your team. In addition, our experts may assist you with case follow-ups and case completion on your behalf. We go above and above to help your customers and patients in every way that we possibly can.
Medical Information Database
Biomapas Medical Information specialists collecting, manage, and provide real-time oversight of so you can make informed decisions and improved compliance. We streamline our efforts with our adverse event management team and apply with role-based assignment and routing of safety cases for follow-up and medical review.
Medical Communication
Pharmaceutical firms must continuously communicate and educate healthcare professionals to enhance patient care. The traditional definition of Medical Information refers to collecting data, generating the content, responding, and evaluating findings. At Biomapas, Medical Communication represents strategic strategies for presenting and disseminating information to your intended audience.
Analytics & Insights
What value can Medical Information activities provide a business to turn a legal obligation into a competitive advantage? Biomapas' Medical Information services provide enormous insights and results with significant strategic potential for pharmaceutical companies and should be a key partner in designing patient experiences. These insights help recognize emerging themes, indicate early problems, and identify high-impact areas for future medical education.
Integrations
We combine our Medical Information expertise with RA & PV in multi-disciplinary teams. As a result, we can offer our clients a combination of in-house expertise and the tools they need to guarantee patient safety.
Full Product Lifecycle
Since the advent of ATMPs and personalized medications, it has become more challenging to provide Medical Information analytics. Due to a combination of high demand, limited resources and growing difficulties in getting new products on the shelves, operations may be severely impacted by this. We offer efficient solutions to ensure contact centre traffic, triage, and response documents are adequately managed consistently and globally.
Content Creation & Management
Your library of critical documentation can be created entirely or seamlessly added to by Biomapas' medical writing experts, who have extensive expertise in various therapeutic fields and academia.
- Standard Document Creation
- Custom Response and Content Management
- Response Document Maintenance
- Translation and Localization Services
Around The Clock
Our Medical Information staff represents highly-trained experts, which includes multilingual native speakers. In addition, of course, our Contact Center Support is available 24/7/365. Above all, Biomapas aims to put technology first to offer a modern solution that is both high-quality and efficient.
Global & Local Coverage
Because we have local employees throughout all of the European, CIS, EAEU and MENA regions, we have a high level of regional, cultural, and legal knowledge under the Biomapas umbrella. In addition, as a result of our extensive experience in Clinical Research, Regulatory Affairs, Pharmacovigilance, and Medical Information, we can provide comprehensive solutions.

Flexibility and global reach are at the core of our medical information services. Whether you need standalone support or an integrated approach with pharmacovigilance, we deliver tailored solutions with a balance of global oversight and local expertise, ensuring consistency, clarity, and compliance across every region.

Advanced Technology for Smarter MI
We use tools like En3 by Nexentria to ensure inquiries are logged, tracked, and answered efficiently. Automation helps reduce response times and ensures accuracy, even for high inquiry volumes.

Medically Trained Experts
Our team includes industry specialists and medically trained staff who bring the expertise needed to develop complete responses and answer complex questions accurately and confidently.
Native Speakers Worldwide
Accurate communication starts with language. Our team includes native speakers across our global network, ensuring your medical information is delivered clearly and appropriately for local audiences.
Related insights
Whitepaper

Webinar Replay
Explore the advantages of combining these two critical components of drug safety.

Blog
Walk through the steps of launching a medical information scientific service in Europe, our experts provide you with the insights you need to succeed.

Insights delivered to your feed?
Sign up for the Biomapas Bulletin on LinkedIn






